45 phase labels in chemical equations
› chemistry-solversChemistry Problem Solver Online | Chemistry Homework Help ... If those strange equations a chemistry teacher writes on a chalkboard drive you crazy, there’s no need in torturing yourself. Thanks to the digital and technological progress, you can find a chemistry problem solver for any occasion. Here are some you can use. Chem Wiz; pH Calculator; Balancing Chemical Equations; Mole Calculator Electrochemcal Impedance Spectroscopy (EIS) Basics 24.2.2022 · The impedance is proportional to the frequency-dependent voltage and frequency-dependent current , where is the angular frequency of an oscillating sine wave. The definition of impedance comes from electrical circuits, and as a result, voltage is commonly used to define impedance. However, in electrochemical impedance spectroscopy, we will switch from using …
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).

Phase labels in chemical equations
Gibbs' Phase Rule: Where it all Begins - Teaching Phase Equilibria P independent equations (of the Gibbs-Duhem form) that describe the energetics of the system--one equation for each phase. In mathematical terms, the variance (F) is determined by the difference between (C+2) variables and (P) equations. Thus, F = C + 2 - P or as originally written, P + F = C + 2. A more extensive derivation of the phase rule ... System - Wikipedia A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its environment, is described by its boundaries, structure and purpose and expressed in its functioning. Systems are the subjects of study of systems theory and other systems sciences en.wikipedia.org › wiki › Scientific_lawScientific law - Wikipedia Scientific laws or laws of science are statements, based on repeated experiments or observations, that describe or predict a range of natural phenomena. The term law has diverse usage in many cases (approximate, accurate, broad, or narrow) across all fields of natural science (physics, chemistry, astronomy, geoscience, biology).
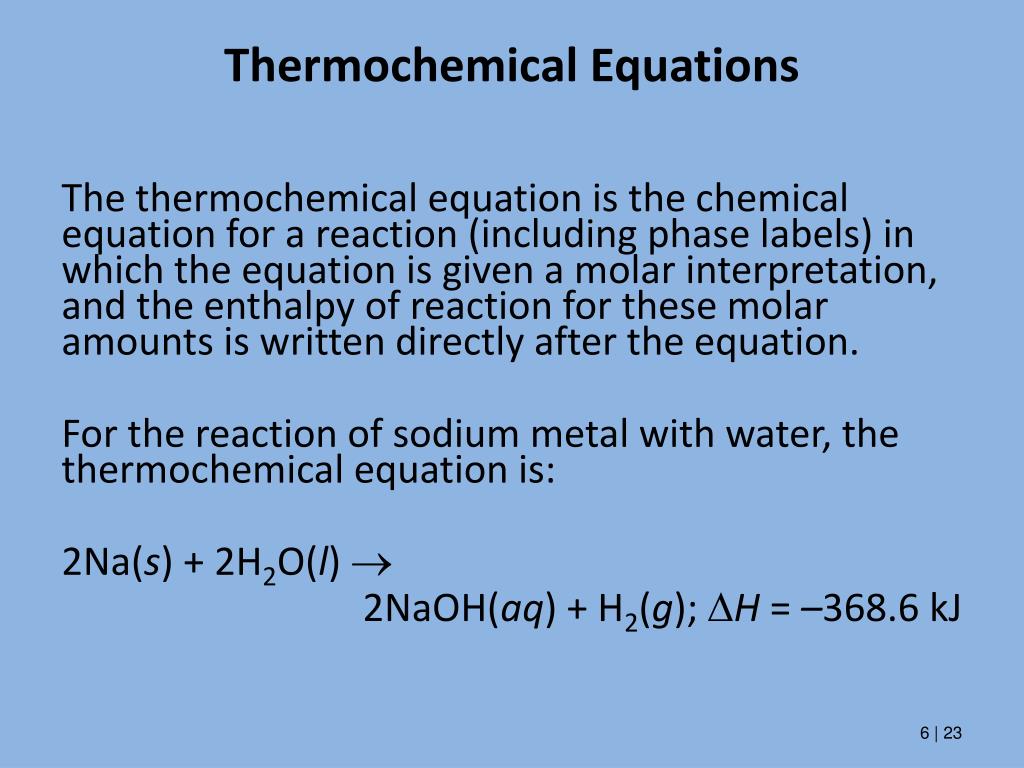
Phase labels in chemical equations. Answered: Net Ionic Equation for the following… | bartleby Q: Net Ionic Equation for the following (include phase labels and ion charges) Na2CO3 + AgNO3 And… A: The sodium carbonate (Na2CO3) reacts with silver nitrate (AgNO3) to form silver carbonate as the… Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3. pubs.acs.org › doi › 10Artificial Intelligence in Meta-optics | Chemical Reviews Recent years have witnessed promising artificial intelligence (AI) applications in many disciplines, including optics, engineering, medicine, economics, and education. In particular, the synergy of AI and meta-optics has greatly benefited both fields. Meta-optics are advanced flat optics with novel functions and light-manipulation abilities. The optical properties can be engineered with a ... A thermochemical equation is a balanced chemical reaction equation ... A thermochemical equation is a balanced chemical reaction equation (including phase labels) with the enthalpy of reaction value written directly after the equation THERMOCHEMICAL EQUATIONS A reaction that produces heat is referred to as exothermic. For exothermic reactions, enthalpy values are assigned a negative value (-DH).
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways Droplet-based microfluidics - Wikipedia Droplet-based microfluidics manipulate discrete volumes of fluids in immiscible phases with low Reynolds number and laminar flow regimes. Interest in droplet-based microfluidics systems has been growing substantially in past decades. Microdroplets offer the feasibility of handling miniature volumes (μl to fl) of fluids conveniently, provide better mixing, encapsulation, sorting, … Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ... Answered: Complete and balance the following… | bartleby Solution for Complete and balance the following molecular equations (indicate the relevant phase labels): LICI (s) + HSO4 (aq) → NH. (aq) + OH(aq) → AgNOs (aq) ... Using chemical equations, show how the triprotic acid H₂PO4 ionizes in water. Phases are optional. ...
Solved Part 7: Aqueous Chemistry...Ionic Equations 7. In | Chegg.com In many aqueous chemical reactions, there are ions that are not involved in the chemical change but serve to deliver the ions that are involved. hen a compound in a chemical equation has an aqueous label, and is ionic, it consists of the constituent ions separated in solution. We represent the ions in a complete This question hasn't been solved yet diffeq.sciml.ai › stable › tutorialsOrdinary Differential Equations · DifferentialEquations.jl AutoVern7(Rodas5()) handles both stiff and non-stiff equations in a way that's efficient for high accuracy. Tsit5() for standard non-stiff. This is the first algorithm to try in most cases. BS3() for fast low accuracy non-stiff. Vern7() for high accuracy non-stiff. Rodas4() or Rodas5() for small stiff equations with Julia-defined types, events ... End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Scientific law - Wikipedia Scientific laws or laws of science are statements, based on repeated experiments or observations, that describe or predict a range of natural phenomena. The term law has diverse usage in many cases (approximate, accurate, broad, or narrow) across all fields of natural science (physics, chemistry, astronomy, geoscience, biology).Laws are developed from data and can be further …
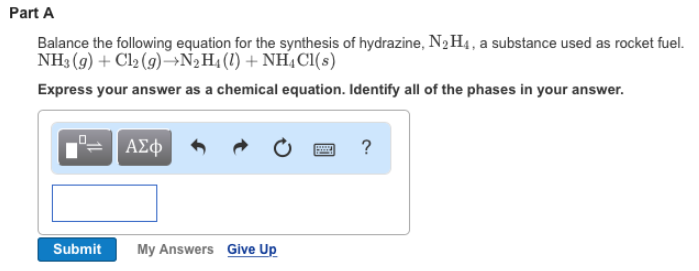
Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? 3.
Ordinary Differential Equations · DifferentialEquations.jl - SciML AutoVern7(Rodas5()) handles both stiff and non-stiff equations in a way that's efficient for high accuracy. Tsit5() for standard non-stiff. This is the first algorithm to try in most cases. BS3() for fast low accuracy non-stiff. Vern7() for high accuracy non-stiff. Rodas4() or Rodas5() for small stiff equations with Julia-defined types, events ...

Phase_Diagram_WorksheetL1 - Phase Diagram Worksheet For each of the questions on this worksheet ...
en.wikipedia.org › wiki › SystemSystem - Wikipedia A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its environment, is described by its boundaries, structure and purpose and expressed in its functioning.
The Chemical Equation | Introductory Chemistry - Lumen Learning Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2 NaHCO3(s) →200°C Na2CO3(s) + CO2(g) + H2O (ℓ) Key Takeaways
Phase Transitions: Melting, Boiling, and Subliming Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows: H 2 O (s) → H 2 O (ℓ) No chemical change is taking place; however, a physical change is taking place. Heating Curves
The Chemical Equation | Introductory Chemistry - 1st Canadian Edition In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ...

Point To Point Diagrams - 2 4 Phase Diagrams Chemistry Libretexts / Both numbers tell us about ...
› questions-and-answers › completeAnswered: Complete and balance the following… | bartleby Transcribed Image Text: Complete and balance the following molecular equations (indicate the relevant phase labels) LICI (s) + HSO4 (aq) →→ NH₂ (aq) + OH(aq) → AgNOs (aq) + + (aq) →> NaHCOs (aq) + H* (aq) → 0 0 0. Expert Solution. ... Using chemical equations, show how the triprotic acid H₂PO4 ionizes in water. Phases are optional
What are Chemical Equations? Detailed Explanation, Examples - BYJUS An example of an ionic chemical equation is provided below. Chemical Equation: CaCl 2 + 2AgNO 3 → Ca (NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3- → Ca 2+ + 2NO 3- + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ ( calcium ion ...
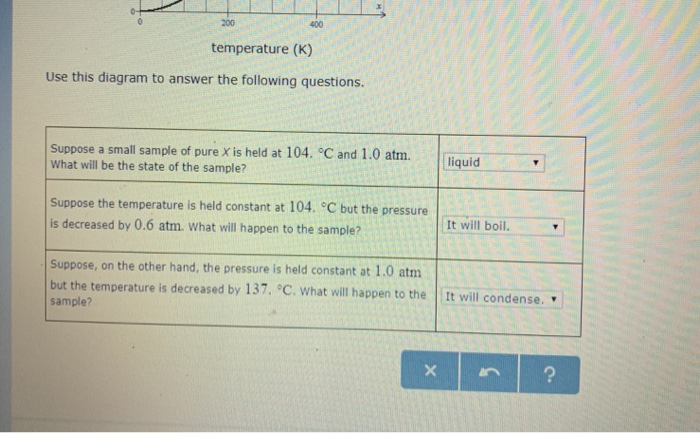
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution
en.wikipedia.org › wiki › Droplet-based_MicrofluidicsDroplet-based microfluidics - Wikipedia Surfactants play an important role in droplet-based microfluidics. The main purpose of using a surfactant is to reduce the interfacial tension between the dispersed phase (droplet phase, typically aqueous) and continuous phase (carrier liquid, typically oil) by adsorbing at interfaces and preventing droplets from coalescing with each other, therefore stabilizing the droplets in a stable ...
Chemistry Problem Solver Online - Online Homework Help for … If those strange equations a chemistry teacher writes on a chalkboard drive you crazy, there’s no need in torturing yourself. Thanks to the digital and technological progress, you can find a chemistry problem solver for any occasion. Here are some you can use. Chem Wiz; pH Calculator; Balancing Chemical Equations; Mole Calculator
Solved 2. Chemical equations can also be used to represent | Chegg.com Write a chemical reaction for the freezing of water, including the proper phase labels. 3. Explain why 4Na (s) + 2C12 (9) - 4NaCl (s) should not be considered a proper chemical equation. 4. Explain why H2 (g) + 1/2O2 (g) → H20 (1) should not be considered a proper chemical equation. 5. Does Question: 2.
The Chemical Equation - GitHub Pages In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ...
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust.
End-of-Chapter Material | Introductory Chemistry - Lumen Learning Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Tinker, Taylor, Builder, Nexus (Worm/Eclipse Phase ... - SpaceBattles 10.3.2022 · Recent threadmarks Chapter 3-4 - Format, part 4 - In which accidents happen. New Chapter 3-3 - Format, part 3 - In which the shit hits the fan. New Chapter 3-2 - Format, part 2 - In which Taylor finally meets her heroes. New Chapter 3-1 - Format, part 1 - wherein Taylor gets a move on New Interlude 3 - Protectorate & Undersiders - wherein several people try to figure …
Artificial Intelligence in Meta-optics | Chemical Reviews Recent years have witnessed promising artificial intelligence (AI) applications in many disciplines, including optics, engineering, medicine, economics, and education. In particular, the synergy of AI and meta-optics has greatly benefited both fields. Meta-optics are advanced flat optics with novel functions and light-manipulation abilities. The optical properties can be engineered with a ...







Post a Comment for "45 phase labels in chemical equations"